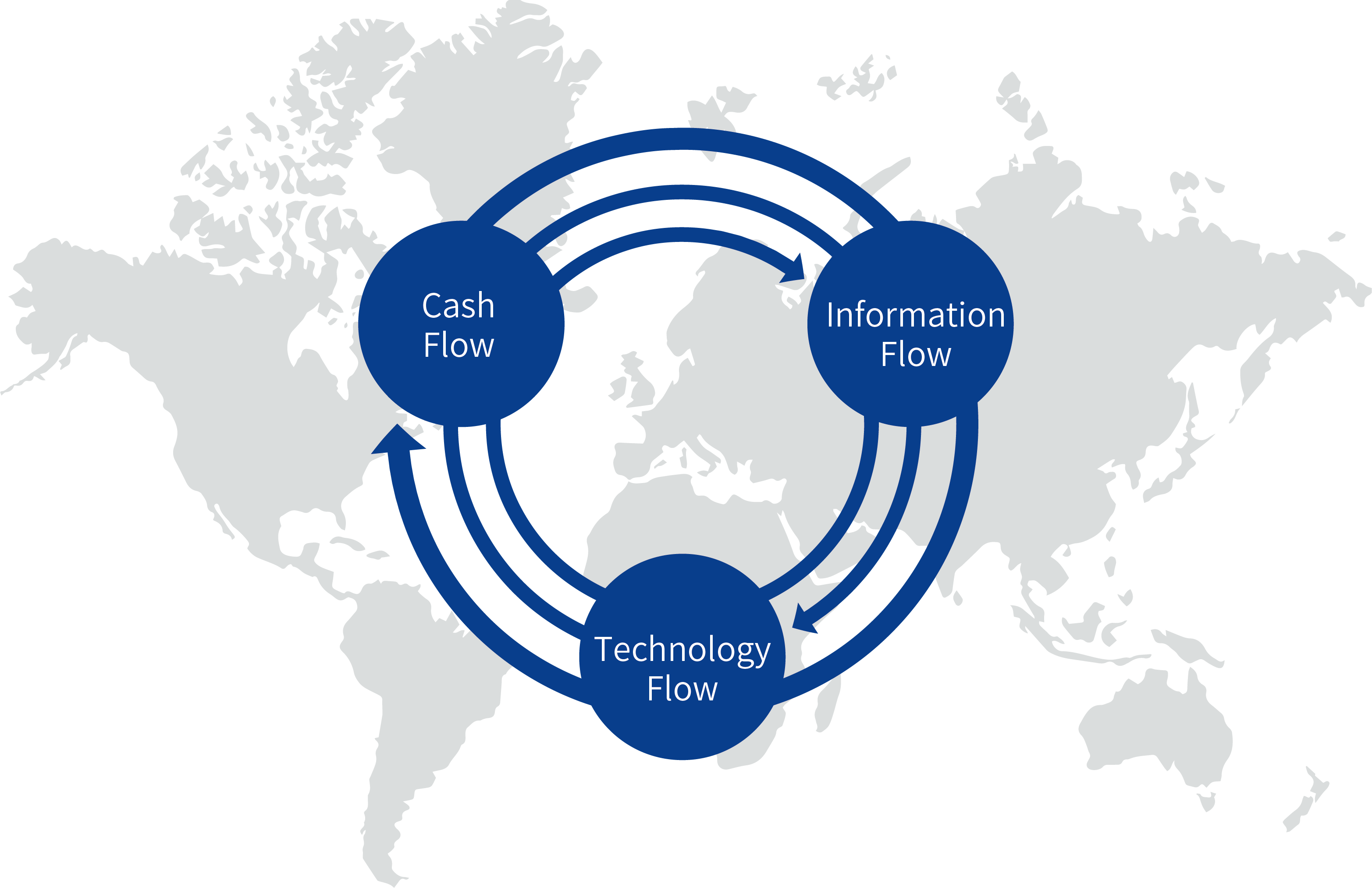
Our key business activities are based on big data and data mining, alongside financial and Sci-tech services as well as related knowledge transfer processes to break through the current global information barrier and geo-relation block. Through achieving this, we aim to enhance the circulation and cycling of the global information flow, technology flow and financial flow within the rehabilitation and nursing sector. We endeavor to speed up the commercialisation of due application sectors in China.
CRNTI team is composed of financial professionals, industry experts, researchers and practitioners alongside academic scholars. Through implementation of our unique and effective domestic and overseas knowledge transfer mode and mechanism, we are able to effectively resolve the last mile issue of transforming knowledge into commercialized products.
Apply big data and data mining techniques to conduct academic due diligence and market investigation, to explore the potential research and research groups from global higher education bodies for knowledge transfer services. Furthermore, by verifying the authenticity of both academic research and industry pain point, to match the gaps in due research and market needs, to conduct the project initiation and evaluation.
CRNTI will provide (i) incubation docking to China, (ii) seed funding for early stage development of all qualified projects, (iii) scheme of shareholder structure and (iv) risk management assistance to the relevant startup companies.
Assist the investee company in (i) patent mining & patent portfolio for related projects, (ii) registration of high-tech enterprises in China, (iii) acquisition of frontier technology and management talents, (iv) deployment of project specialists to provide enterprises with full-process Sci-tech services for Funding applications in China.
Our project management specialists can assist in (i) post-investment services for the due project, (ii) prototype fabrication, (iii) medical equipment clinical trials and CFDA registration, and (iv) simultaneously providing relevant market exploration assistance and financial advisory services.

